How Metamaterial Design Could Give Interventional Cardiologists Finer Control in Complex Heart Surgeries
Coronary artery disease afflicts 18 million Americans, and some of the most challenging cases continue to remain stubbornly intractable, causing a significant economic strain. Frail patients who are poor candidates for bypass surgery with tortuous, calcified vessels rely on stent implantation through percutaneous coronary intervention (PCI). Cardiologists require enhanced catheter support and steerability to access these complex lesions, yet antiquated technology undermines their efforts, leaving physicians to contend with instruments ill-suited to contemporary demands.
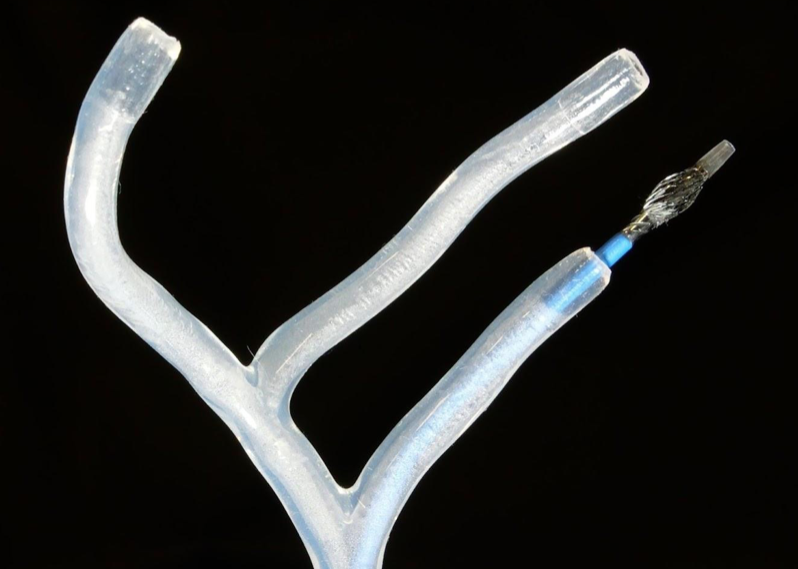
The team is testing the shape-transforming catheter prototype in a silicone model of blood vessels that replicates human vascular anatomy.
A research project at the University of Washington, led by Aman Garg, Research Engineer/Scientist IV, is developing a novel catheter technology to overcome the limitations of existing devices. The team is developing tubular structures that can alter their mechanical state within the body. These shape transformations are achieved through metamaterials, where geometry, not only bulk material, does the heavy lifting by integrating mechanical intelligence into the device structure. The objective is straightforward: give physicians finer control when and where it is needed, then return to a safe, low-profile configuration for advancement or withdrawal.
What a Shape-transforming Catheter Is
The concept behaves like two devices in one. In its baseline state, the shaft tracks gently and resists catching on delicate tissue. When commanded, the distal tip segment dilates and anchors into the coronary. The change is local, predictable, and controllable, so the physician no longer has to struggle to maintain anatomical stability while preserving vessel perfusion, unlike with a balloon catheter. The segment is then released, and the device returns to baseline.
Mechanically, the behavior is architectural. The team encodes mechanical phases into the geometry of a catheter segment, allowing for switches between “compliant” and “supportive” states without the need for powered actuators or significant changes in outer diameter. In early lab work, a slight, well-placed local shape shift or bias produced measurable gains in controlled stability. The task now is to quantify those gains and understand the cost in terms of tissue interaction.
Soft-robotics Principles at the Clinical Scale
The effort borrows from soft robotics. Garg and collaborators have developed shape-transforming structures that add degrees of freedom without power-hungry actuation, a design language that can augment medical tubing. The promise is control on demand, without creating complex, hard-to-sterilize, hard-to-miniaturize, or hard-to-trust systems in critical care.
For a deeper background, see the related publications in Advanced Intelligent Systems, Science Robotics, and IEEE Xplore, which describe how geometry and layered structures produce multi-state behavior and how these states can be switched in a controlled manner.
What Has Been Tested So Far (Research Stage)
The program is strictly research-stage and focused on preclinical evidence:
- Bench testing. Push and track forces are measured as structures move through representative paths, with attention to peak/average force, capture points, and how profiles change when segments switch state.
- Explant studies. In explant porcine hearts, the team evaluated catheter stability through tight turns and calcified analogs, scoring path bias, success rates, and qualitative operator effort.
- In vivo (planned). An in-vivo swine study is scheduled this quarter to observe coronary stability under physiological conditions. Endpoints include device stability and tissue-interaction assessment through histology studies.
No clinical efficacy claims are made as the studies address a more modest question: Can local, reversible shape change reduce the day-to-day trade-off between “more support” and “more trauma” that interventionalists manage?
Regulatory Perspective: Predicate-based FDA Class II Qualification
The team is evaluating a predicate-based Class II 510(k) pathway to establish regulatory requirements that will inform FDA clearances for any commercialization efforts. The goal is to remain within the risk profile of comparable catheter technology while demonstrating practical usability advantages. The planned dossier emphasizes objective preclinical data (bench → explant → in vivo) and human-factors tasks that mirror high-stakes moments in complex PCI.
Why This Could Matter for Frail or Bypass-ineligible Patients
When anatomy is unforgiving, a small increment of controllability can expand what is possible. A catheter system that dilates and provides coronary stability in a precise region at a precise moment could help physicians cross without resorting to globally stiffer hardware. If that reduces exchanges and manipulation, it may shorten procedures and reduce the risk of harm. The potential patient impact is intuitive: more anatomies become percutaneously treatable, offering options to patients who were previously considered too risky.
Team, Support, and Translation
Garg leads a multi-disciplinary team of clinicians, scientists, and engineers. The research is supported by NIH, NSF, the Washington Research Foundation, and the University of Washington. The program remains focused on evidence that can stand on its own.
Garg’s path informs the work. He previously engineered automotive suspension systems, where predictable, simple mechanisms matter more than clever tricks, and invented medical devices for low-resource hospitals in suburban India, where every added part is a liability. Those experiences bias the group toward designs that are robust, easy to learn, and practical in both laboratory and operating room settings.
What Comes Next
In the coming months, the team plans to complete the in-vivo study and feed the findings back into the engineering loop, refine the metamaterial cell geometry in shape-transforming segments, tune the transitions so they feel natural to operators, and document the boundaries of safe use. Human-factors work will expand in parallel, so training and procedural guidance keep pace with the hardware.
The objective of this research is to establish whether innovative tools can enhance operator control at critical moments without increasing overall risk. Success in this regard will substantiate a novel approach for designing catheter architecture. This standard, if met, has the potential not only to transform percutaneous coronary interventions (PCI) but also to pave the way for a new generation of functional, operator-friendly catheters that can unlock new interventional therapies and improve patient outcomes.